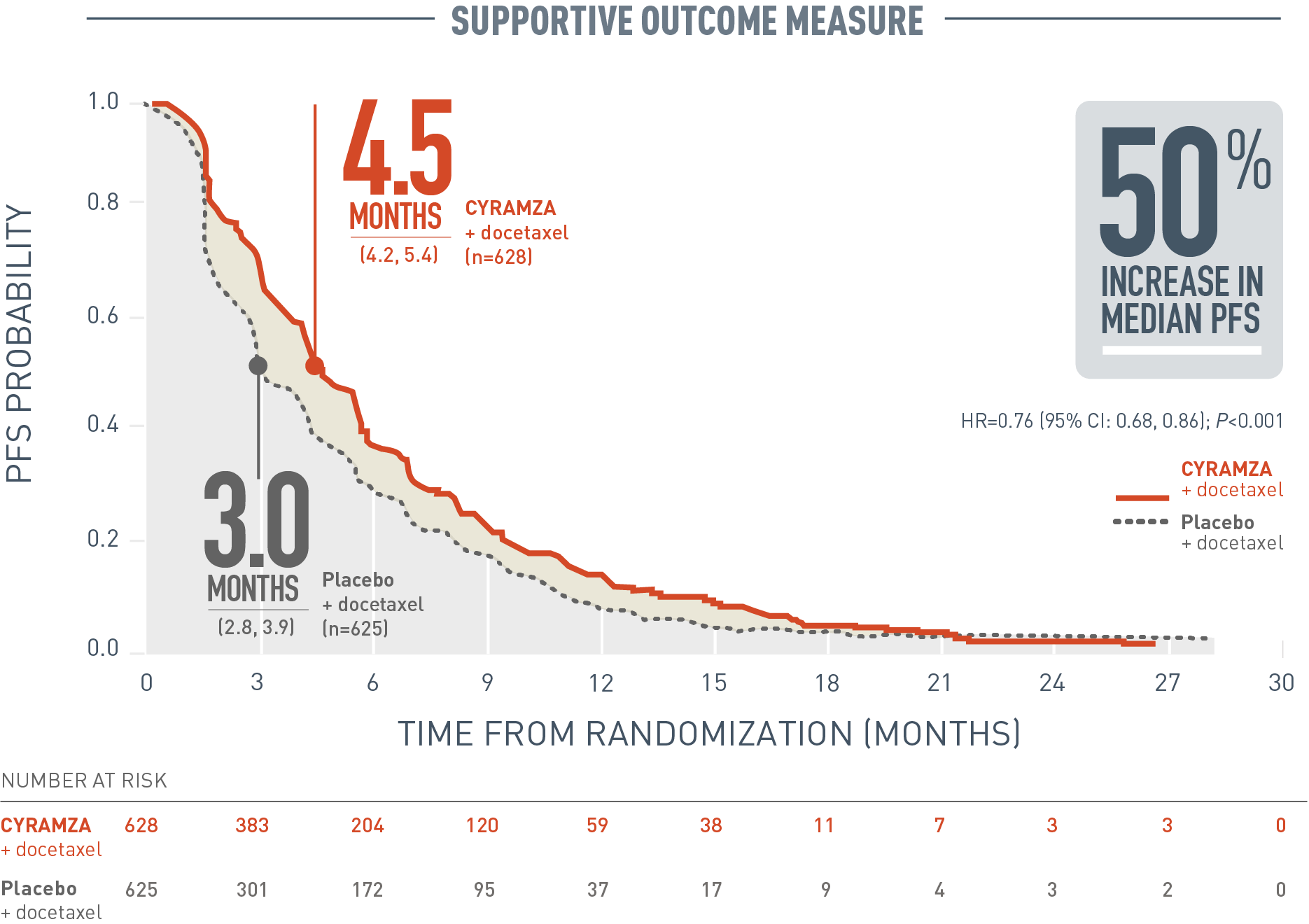
Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study - ScienceDirect
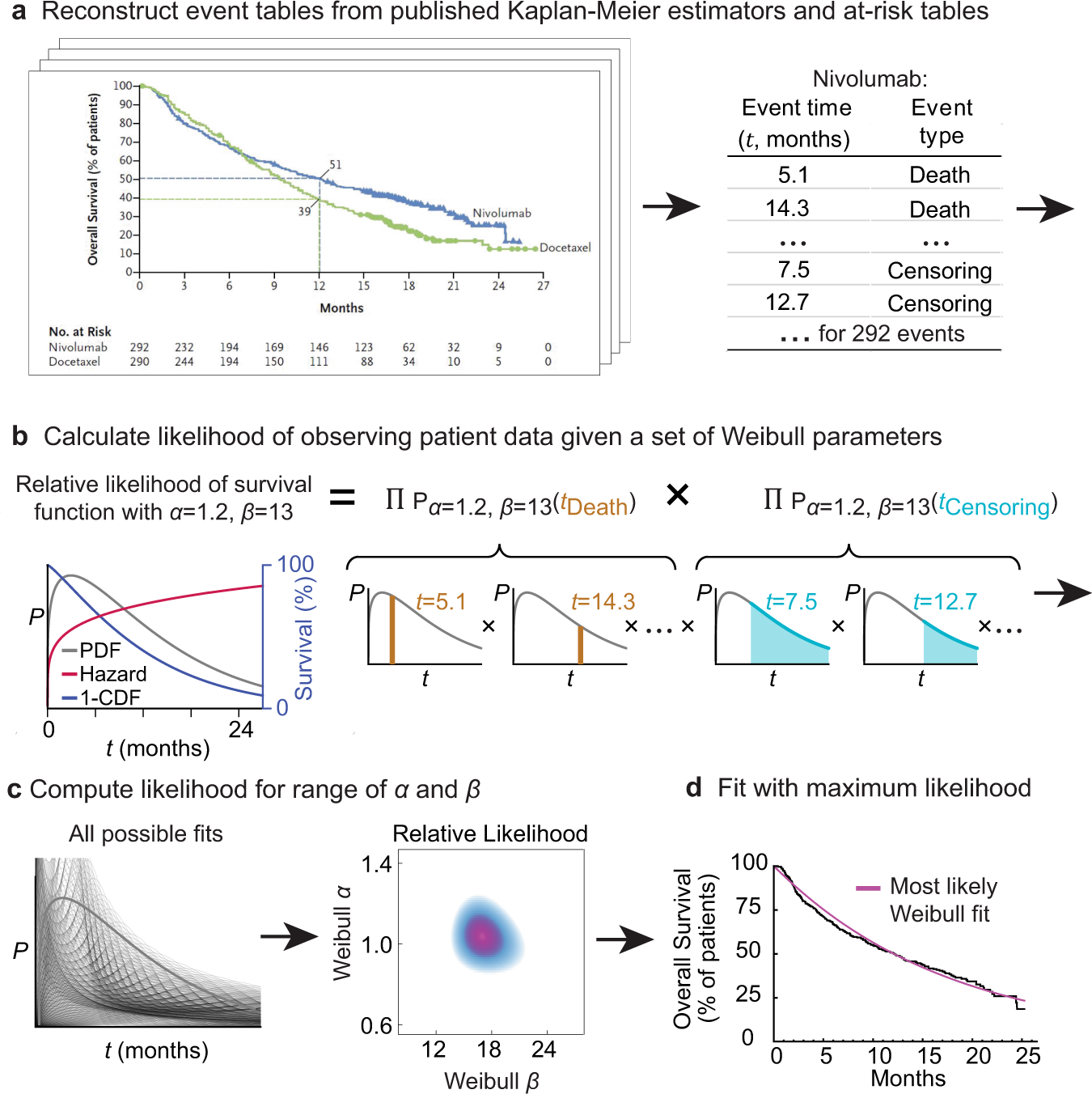
Cancer patient survival can be parametrized to improve trial precision and reveal time-dependent therapeutic effects | Nature Communications

JCM | Free Full-Text | A Clinical Efficacy of PRRT in Patients with Advanced, Nonresectable, Paraganglioma-Pheochromocytoma, Related to SDHx Gene Mutation | HTML
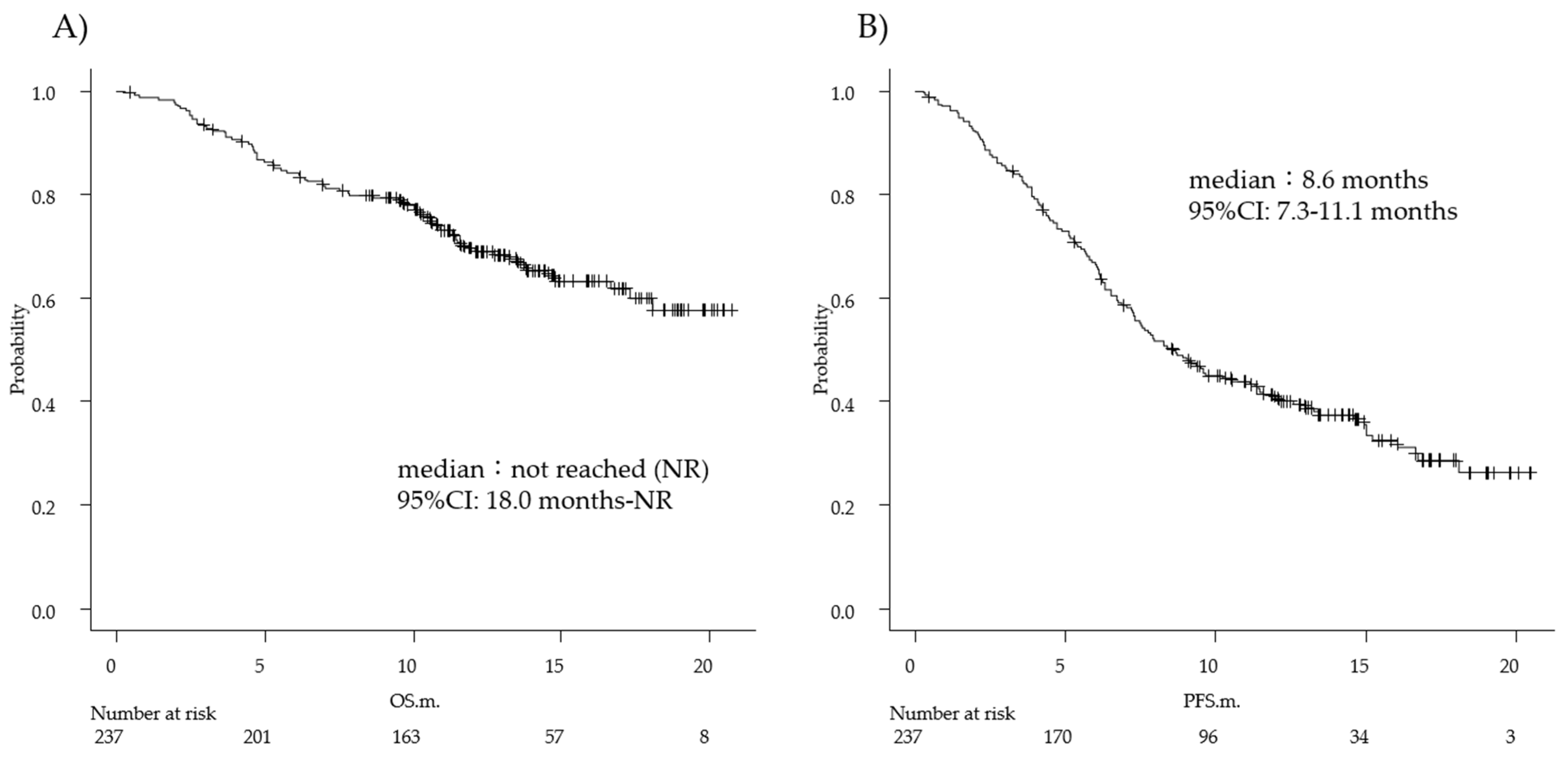
Diagnostics | Free Full-Text | Prognostic Nutritional Index and Lung Immune Prognostic Index as Prognostic Predictors for Combination Therapies of Immune Checkpoint Inhibitors and Cytotoxic Anticancer Chemotherapy for Patients with Advanced Non-Small

NEJM on Twitter: "KEYNOTE-836: In advanced cervical cancer, median PFS was 10.4 months with pembrolizumab and 6 months with placebo. Overall survival at 2 years was 50.4% and 40.4%, respectively. #ESMO21 https://t.co/sZd6SFiGKd
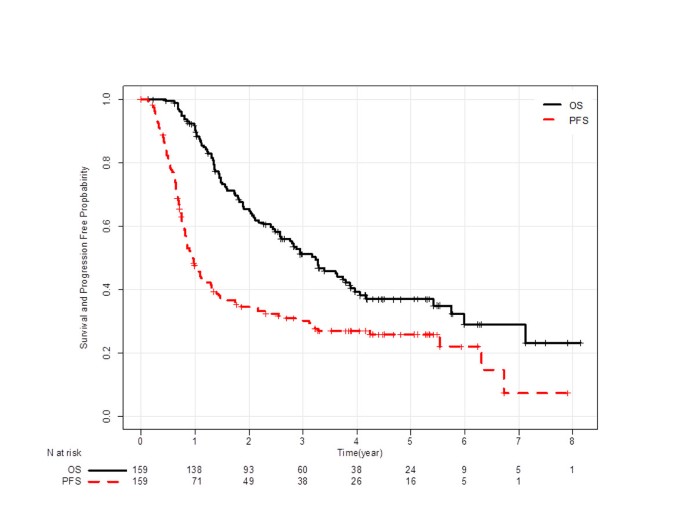
Progression-free survival at 2 years is a reliable surrogate marker for the 5-year survival rate in patients with locally advanced non-small cell lung cancer treated with chemoradiotherapy | BMC Cancer | Full

Exploration of modified progression-free survival as a novel surrogate endpoint for overall survival in immuno-oncology trials | Journal for ImmunoTherapy of Cancer
Median progression-free survival (PFS), duration of response (DOR), and... | Download Scientific Diagram

Progression-free survival (PFS) and overall survival (OS) time. Median... | Download Scientific Diagram

Kaplan–Meier plot of progression-free survival Median progression-free... | Download Scientific Diagram
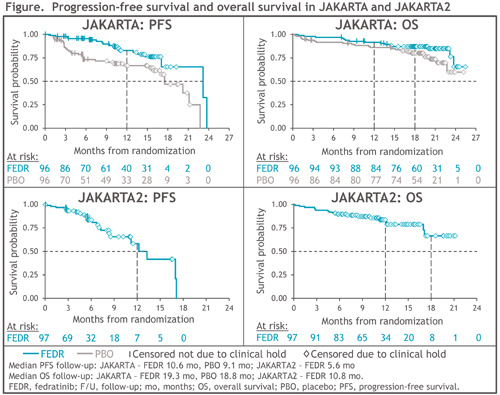
OVERALL AND PROGRESSION-FREE SURVIVAL IN PATIENTS TREATED WITH.... EHA Library. Harrison C. Jun 9 2021; 324611

a) Median progression-free survival (PFS) to second-line (SL) therapy.... | Download Scientific Diagram

Median overall survival (OS) and progression-free survival (PFS) in a... | Download Scientific Diagram

Percentage Progression-free survival and overall survival on treatment.... | Download Scientific Diagram
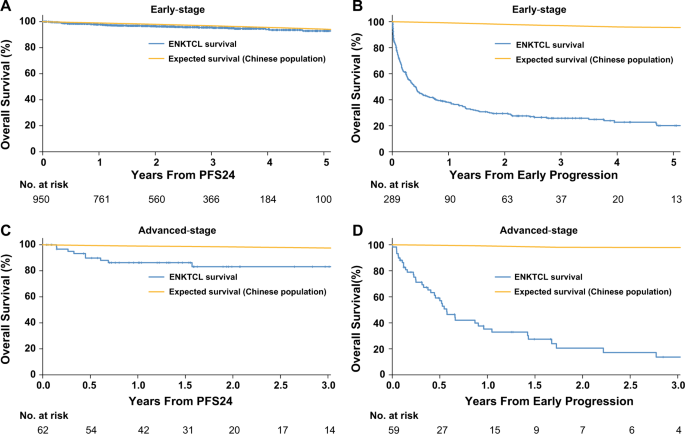
Progression-free survival at 24 months and subsequent survival of patients with extranodal NK/T-cell lymphoma: a China Lymphoma Collaborative Group (CLCG) study | Leukemia
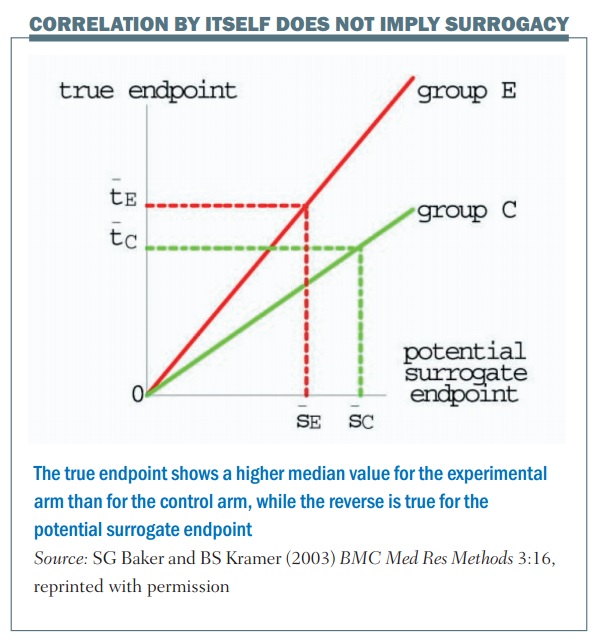
Are progression-free and disease-free survival the new gold standard for cancer trials? | Cancer World Archive