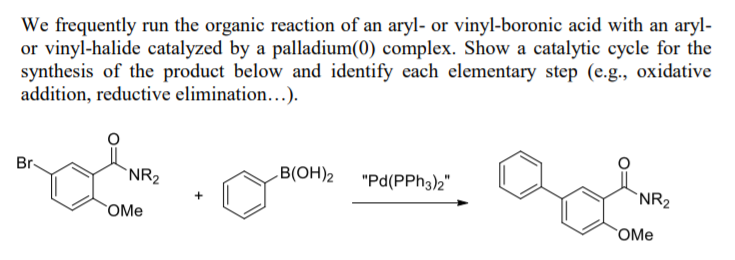
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML
Scheme 1 Synthesis of the trinuclear (1) and mononuclear (2) palladium... | Download Scientific Diagram

Olefin Dimerization and Isomerization Catalyzed by Pyridylidene Amide Palladium Complexes - Organometallics - X-MOL

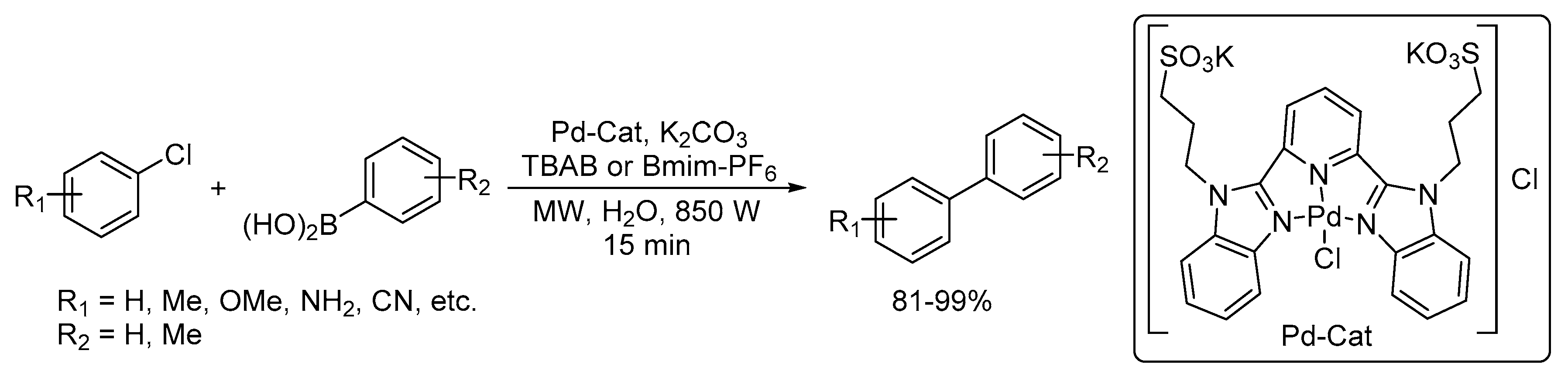
Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds
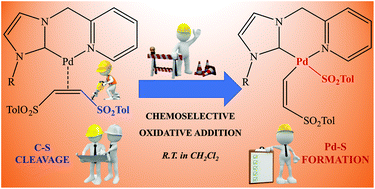
Chemoselective oxidative addition of vinyl sulfones mediated by palladium complexes bearing picolyl-N-heterocyclic carbene ligands. - Dalton Transactions (RSC Publishing)

Figure 1 | Use of a recyclable poly( N -vinyl carbazole) palladium(II) complex catalyst: Heck cross-coupling reaction under phosphine-free and aerobic conditions | SpringerLink

Palladium: Organometallic Chemistry - William Suggs - - Major Reference Works - Wiley Online Library
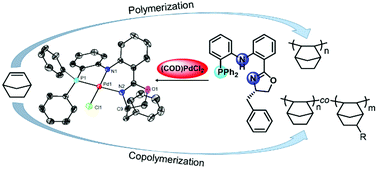
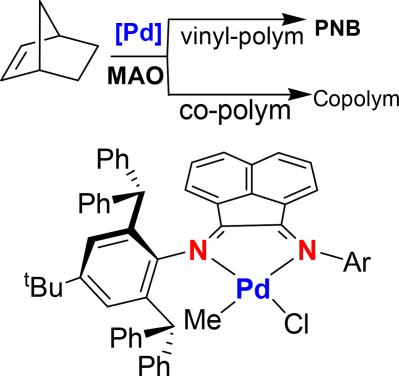
Synthesis of nickel and palladium complexes with diarylamido-based unsymmetrical pincer ligands and application for norbornene polymerization - Dalton Transactions (RSC Publishing)
Vinyl homo/copolymerization of norbornene and ethylene using sterically enhanced 1,2‐bis(arylimino)acenaphthene–palladium precatalysts - J. Polym. Sci. A Polym. Chem. - X-MOL

Palladium(0)/benzoic acid catalysis merges sequences with D2O-promoted labelling of C–H bonds - Chemical Science (RSC Publishing)

Frontiers | Key Mechanistic Features in Palladium-Catalyzed Methylcyclopropanation of Norbornenes With Vinyl Bromides: Insights From DFT Calculations | Chemistry

Highly thermo-stable and electronically controlled palladium precatalysts for vinyl homo/co-polymerization of norbornene-ethylene - ScienceDirect

Proposed mechanistic pathway for the palladium-catalyzed formylation of... | Download Scientific Diagram

Highly active nickel(II) and palladium(II) complexes bearing N,N,P tridentate ligand for vinyl addition polymerization of norbornene - ScienceDirect
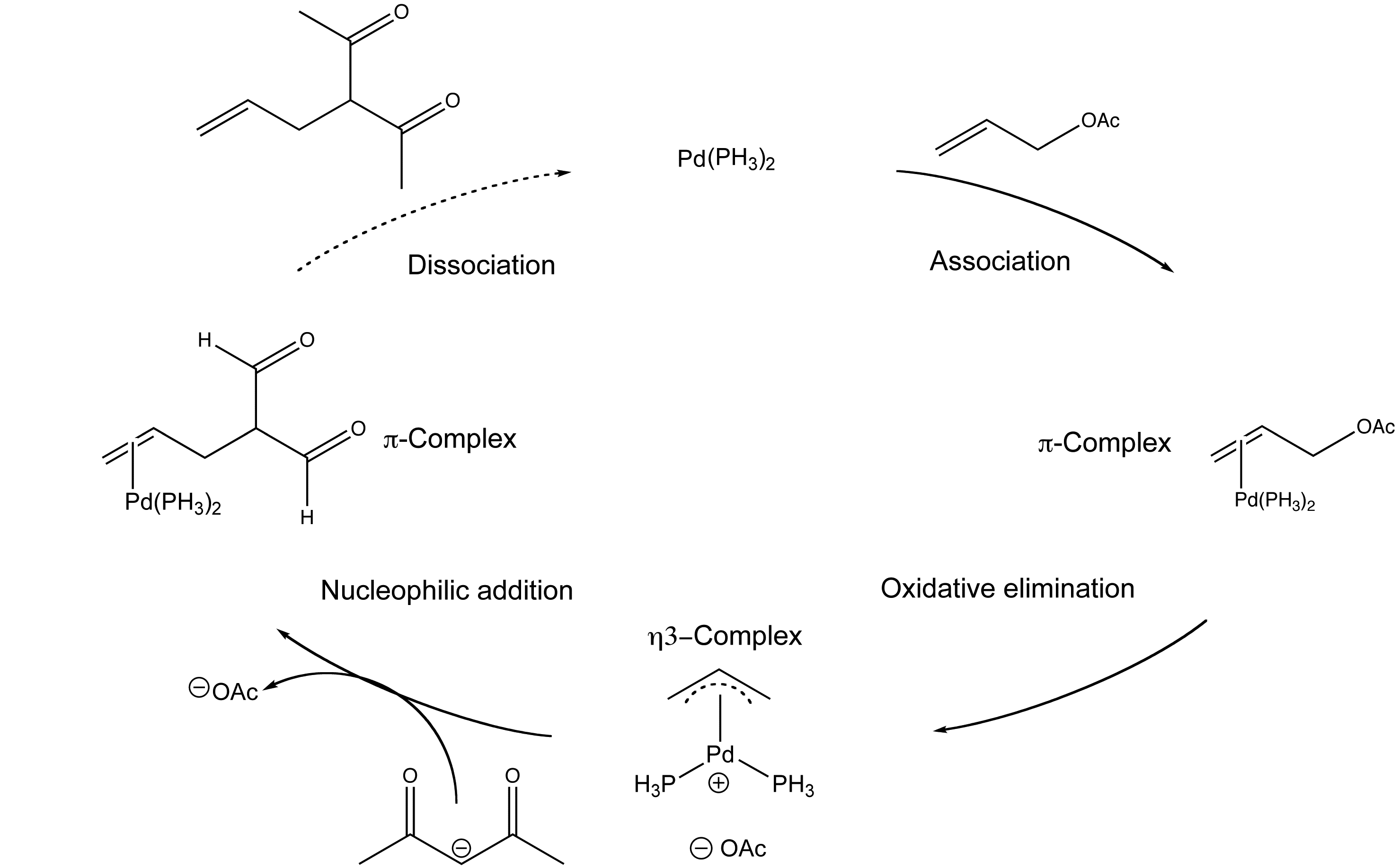
Organopalladium Chemistry - Palladium-catalysed nucleophilic allylic substitution of functionalised compounds
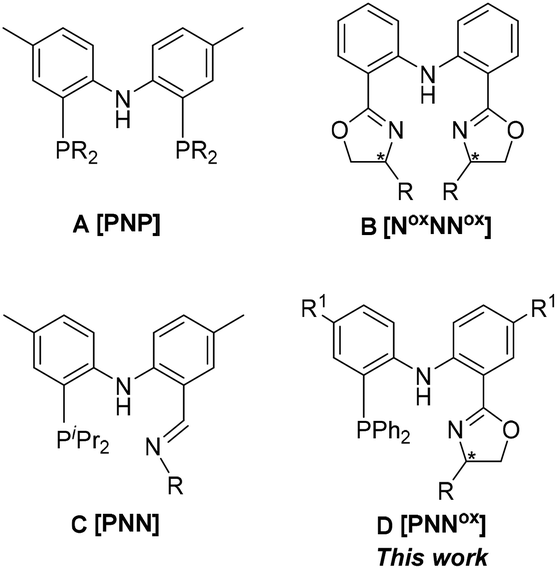
Synthesis of nickel and palladium complexes with diarylamido-based unsymmetrical pincer ligands and application for norbornene polymerization - Dalton Transactions (RSC Publishing)