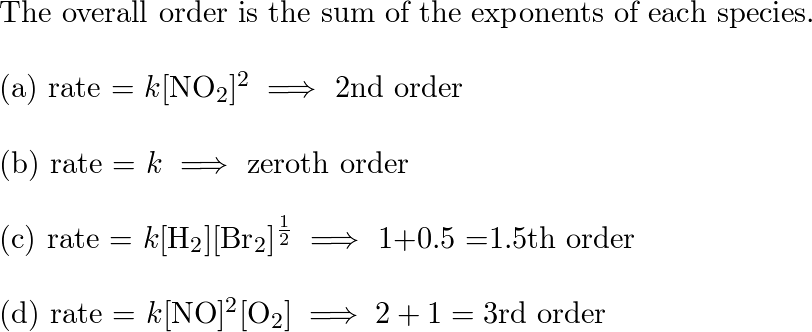![SOLVED: Classify each rate law based on whether the reaction is first-order; second-order; O third-order overall: First-order Second-order Third-order Answer Bank rate k[HCN] rate k[O] [NO] [NzE rate [BFs] [NH,] rate kINO]? SOLVED: Classify each rate law based on whether the reaction is first-order; second-order; O third-order overall: First-order Second-order Third-order Answer Bank rate k[HCN] rate k[O] [NO] [NzE rate [BFs] [NH,] rate kINO]?](https://cdn.numerade.com/ask_previews/7a671ad0-bb32-4650-a5e2-0dc410a20fb1_large.jpg)
SOLVED: Classify each rate law based on whether the reaction is first-order; second-order; O third-order overall: First-order Second-order Third-order Answer Bank rate k[HCN] rate k[O] [NO] [NzE rate [BFs] [NH,] rate kINO]?
![SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order](https://cdn.numerade.com/ask_previews/bea16924-e043-4592-9ba1-7c64b3c6ae29_large.jpg)
SOLVED: What is the overall reaction order for the reaction that has the rate law Rate = k[O2][NO]2? zero order first order second order third order
![SOLVED: Question 3 (1 point) If m=-1 and n-2,then the overall order of reaction is: Question 4 (3 points) Experiment Initial Rate (M s-1) [NOz] (M) A 0.0050 0.10 B 0.0800 0.40 SOLVED: Question 3 (1 point) If m=-1 and n-2,then the overall order of reaction is: Question 4 (3 points) Experiment Initial Rate (M s-1) [NOz] (M) A 0.0050 0.10 B 0.0800 0.40](https://cdn.numerade.com/ask_previews/80ab0b03-b365-4adf-aecd-bfc5fcfa3766_large.jpg)
SOLVED: Question 3 (1 point) If m=-1 and n-2,then the overall order of reaction is: Question 4 (3 points) Experiment Initial Rate (M s-1) [NOz] (M) A 0.0050 0.10 B 0.0800 0.40

Amazon.com: Self Cleaning Cat Litter Box, DECEMPADS Automatic Cat Litter Box Safety Protection Extra Large Cabin Weight Sensor APP Control Timer No Scooping Smart Cat Litter Box Washable Cleaning Cabin : Pet
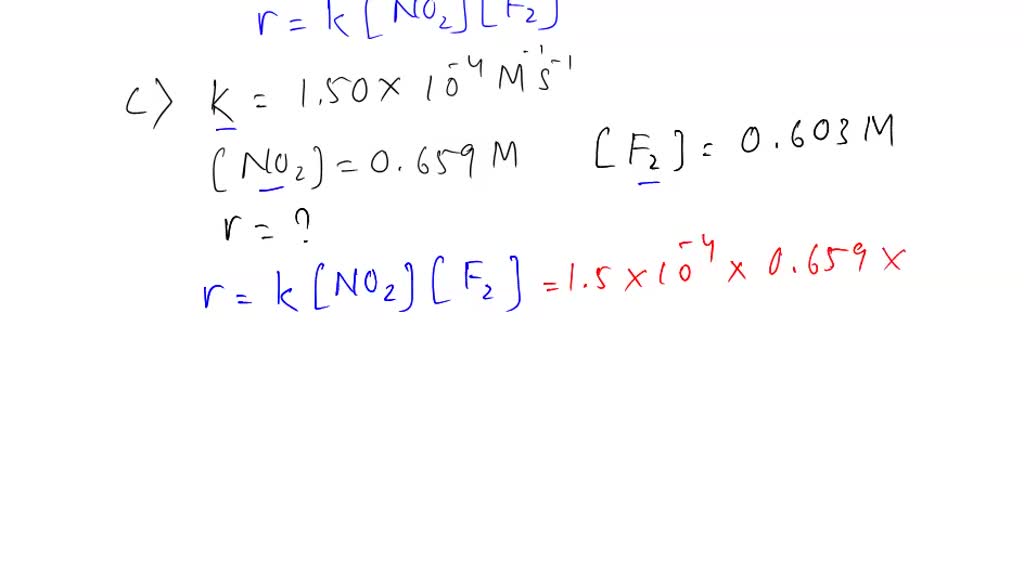
SOLVED: A. The oxidation of nitrogen monoxide by oxygen at 25 oC 2 NO + O2 2 NO2 is second order in NO and third order overall. Complete the rate law for

Amazon.com: NeuType Full Length Mirror 43"x16" Large Mirror Bedroom Locker Room Standing Hanging Mirror Dressing Mirror, Black(No Stand) : Home & Kitchen
![SOLVED: Determine the overall order of the reaction from the following data: Experiment [NO] (M) [Cl2] (M) Rate (M/s) 1 0.0300 0.0100 3.4 × 10-4 2 0.0150 0.0100 8.5 × 10-5 3 0.0150 0.0400 3.4 × 10-4 SOLVED: Determine the overall order of the reaction from the following data: Experiment [NO] (M) [Cl2] (M) Rate (M/s) 1 0.0300 0.0100 3.4 × 10-4 2 0.0150 0.0100 8.5 × 10-5 3 0.0150 0.0400 3.4 × 10-4](https://cdn.numerade.com/ask_previews/62ad3eca-8109-4f88-b404-25181f09255b_large.jpg)
SOLVED: Determine the overall order of the reaction from the following data: Experiment [NO] (M) [Cl2] (M) Rate (M/s) 1 0.0300 0.0100 3.4 × 10-4 2 0.0150 0.0100 8.5 × 10-5 3 0.0150 0.0400 3.4 × 10-4

Amazon.com: NeuType Full Length Mirror 55"x16" Large Mirror Bedroom Locker Room Standing Hanging Mirror Dressing Mirror, Black(No Stand) : Home & Kitchen
![SOLVED: What is the overall order of the reaction below CO (g) + NO2 (g) -> CO2 (g) + NO (g) if it proceeds via the following rate expression? Rate = k[CO][NO2] SOLVED: What is the overall order of the reaction below CO (g) + NO2 (g) -> CO2 (g) + NO (g) if it proceeds via the following rate expression? Rate = k[CO][NO2]](https://cdn.numerade.com/ask_previews/181cde86-7f03-48fb-804d-522fe5d05acf_large.jpg)



![16.1 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube 16.1 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/9sMFJMuZzmg/maxresdefault.jpg)