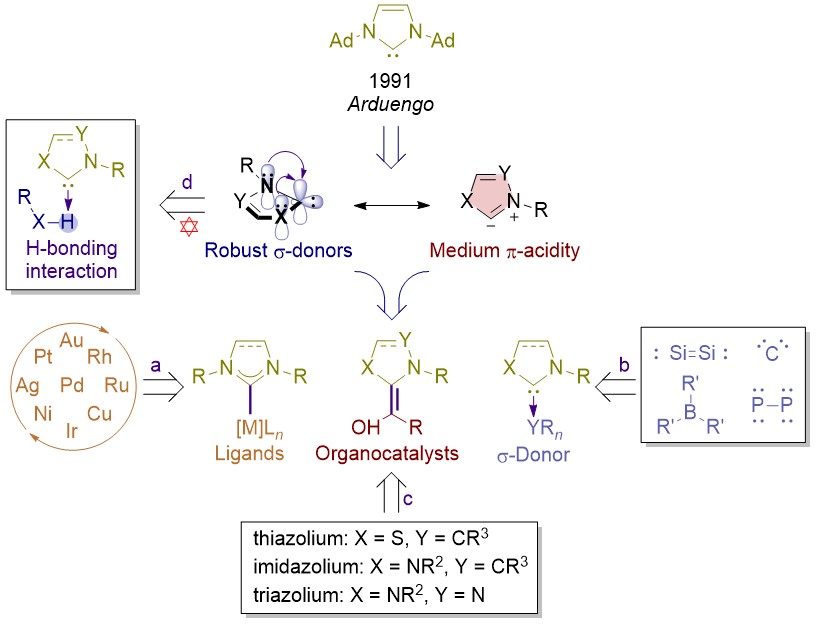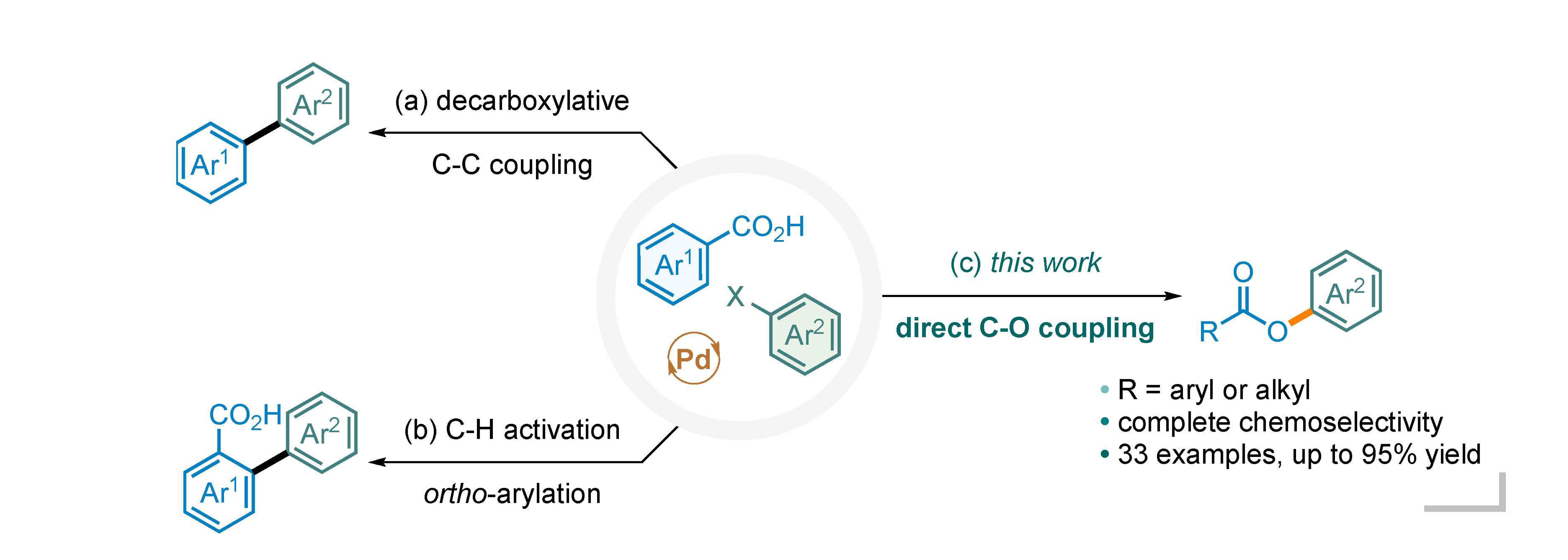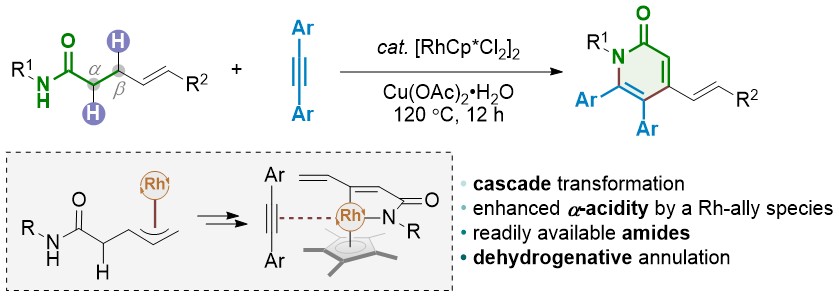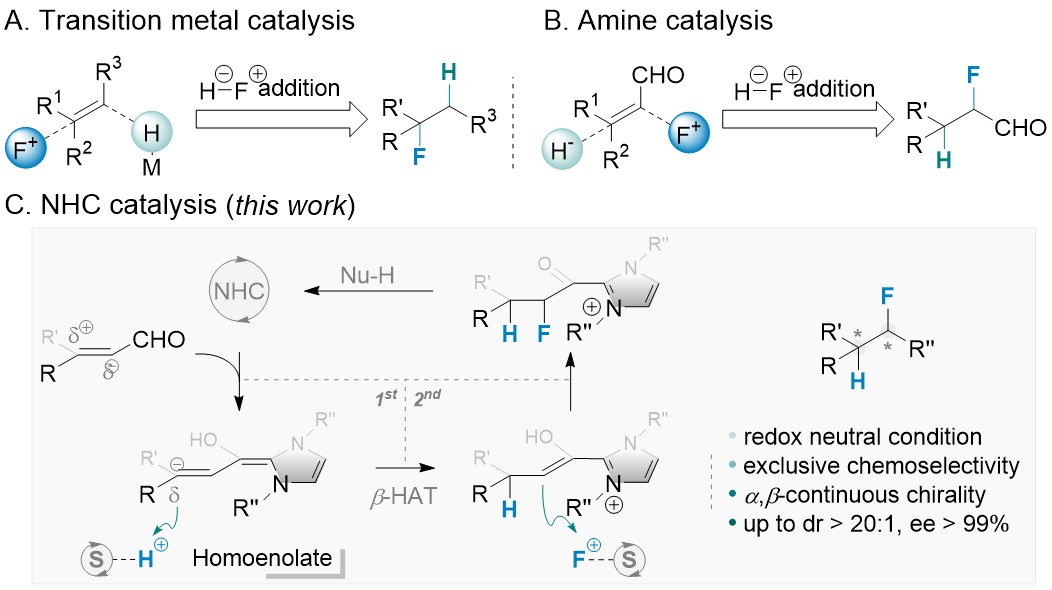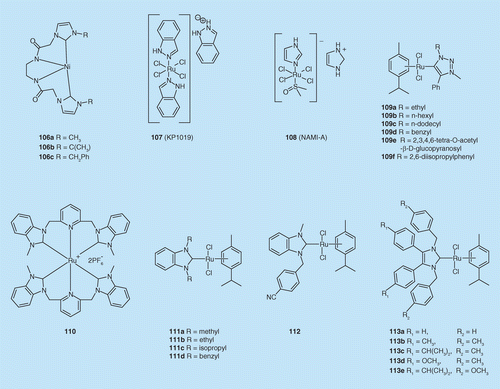
N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs | Future Medicinal Chemistry

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

Recent advances in the catalytic applications of GO/rGO for green organic synthesis in: Green Processing and Synthesis Volume 9 Issue 1 (2020)

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

PDF) Ultra‐small sugar‐substituted N‐heterocyclic carbene‐protected palladium nanoparticles and catalytic activity

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

Pd nanoparticles stabilized on the Schiff base-modified boehmite: Catalytic role in Suzuki coupling reaction and reduction of nitroarenes - ScienceDirect

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

PDF) ChemInform Abstract: Sulfur-Functionalized N-Heterocyclic Carbene Complexes of Pd(II): Syntheses, Structures and Catalytic Activities

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -